Members
Hiroaki Kinoh

belong to
Kataoka-Kina Lab.
employment position or rank
Deputy Lab Director/Principal Investigator
(university) degree
Doctor of Science
uniform resouce locator
Research (research keywords)
(cancer therapy, nanomicelles, cancer immunity, aging control)
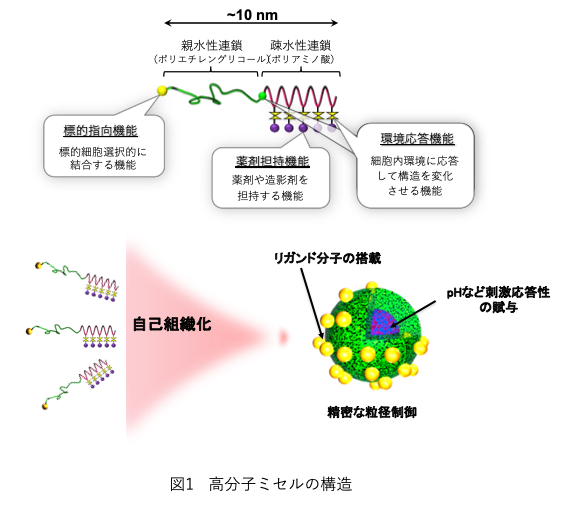
Three trials of polymeric micelles encapsulating anticancer drugs are ongoing in Phase II. In addition to anticancer drug micelles, a new physician-initiated clinical trial for siRNA delivery system (DDS) started last year (2020), and trials are underway to commercialize polymeric polymers as a new DDS for a variety of applications. Recent clinical trials include the combination of cisplatin micelles and Keytruda for head and neck cancer (Phase II), and preclinical studies have shown that the combination of epirubicin micelles and immune checkpoint inhibitors (ICIs) is highly effective in an immune checkpoint inhibitor-resistant brain tumor model. The combination of ICI and micelles has shown dramatically higher anti-tumor effects due to the infiltration of CD8, dendritic cells, and other cells into the cancer. They also report the development of a ligand-loaded delivery system that carries ICI to the brain while avoiding immune-related adverse events (irAE: interstitial pneumonia, etc.) with immune checkpoint inhibitors as a new DDS. Here, we will focus on chemoimmunotherapy using a combination of polymeric micelles and immune checkpoint inhibitors, as well as recent research on delivery systems of immune checkpoint inhibitors to the brain.
Introduction.
Polymeric micelles can be optimized and functionalized for specific purposes such as loading and release control of any drug, precise particle size control in the range of 30-70 nm, and provision of target-directedness and environmental responsiveness by precise design and molecular modification of the block copolymers that comprise the micelles. It is a nano-DDS technology that differs significantly from conventional DDS technologies such as liposomes(1) (Figure 1), and three types of anti-cancer drug-encapsulated micelles and nucleic acid drug-loaded complexes are currently undergoing clinical trials (Figure 2). Anticancer drug-incorporated micelles accumulate in cancer cells through the EPR (enhanced permeability and retention) effect, in which polymeric drugs accumulate in tumors, and release anticancer drugs into cancer cells in response to the cancer environment, showing high antitumor effects. Furthermore, by minimizing the accumulation and release of anticancer drugs to normal cells, it is possible to suppress side effects1). This paper focuses on the research on the combination of polymeric micelles and immune checkpoint inhibitors, which are currently under development and are being tested in clinical trials. ■Chemoimmunotherapy by Combination of Polymeric Micelles and Immune Checkpoint Inhibitors
① Immuno-checkpoint inhibitor (ICI)
Recent rapid progress in tumor immunology research has revealed cancer immune escape mechanisms, and immuno-checkpoint inhibitors (ICIs), which inhibit two auxiliary signals (immune checkpoints) CTLA-4 and PD-1 pathways that suppress T-cell immunity, have been established as a promising treatment for cancer in the clinic. ICIs have been approved for a wide range of cancers (melanoma, non-small cell lung cancer, head and neck cancer, renal cancer, malignant mesothelioma, etc.) and are highly effective, but the following problems have been pointed out. (1) The response rate as a single agent is low (approximately 10-30%) and ineffective for many patients. (2) ICIs have unique immune-related adverse events (irAEs), such as interstitial pneumonia, colitis, hypothyroidism, and liver dysfunction. Many clinical trials of chemo-immunotherapy (CIT), a combination of chemotherapy and immunotherapy, are underway to correct the low response rate.
② Epirubicin micelles and immune response-induced cell death (ICD)
The mechanisms of immune responses to conventional cytotoxic anticancer agents and radiation therapy (RT) are being elucidated and their promising effects are becoming clear. It is now clear that anthracyclines induce immune response-induced cell death (ICD) when cancer cells die and cancer antigens are released, eliciting an immune response that leads tumor cells to cell death. ICD is the death of cancer cells by cytotoxic anticancer drugs or radiotherapy, which results in the expression of CRT (Calreticulin) and the release of HMGB1 (High Mobility Group Box 1) from cancer cells (Eat me signal) the surface of cancer cell membranes, and the release of HMGB1 (High Mobility Group Box 1) from the cancer cells. (7)The ICDs have shown that anthracyclines, including epirubicin, are the most potent inducers of immunity(8) , and have been shown to have reduced side effects and to be highly concentrated in cancer cells. Epirubicin micelles, which have reduced side effects and better accumulation in cancer, are thought to be highly effective for ICD in vivo.
③ Chemo-immunotherapy with epirubicin micelles and ICI dramatically increases anti-tumor efficacy
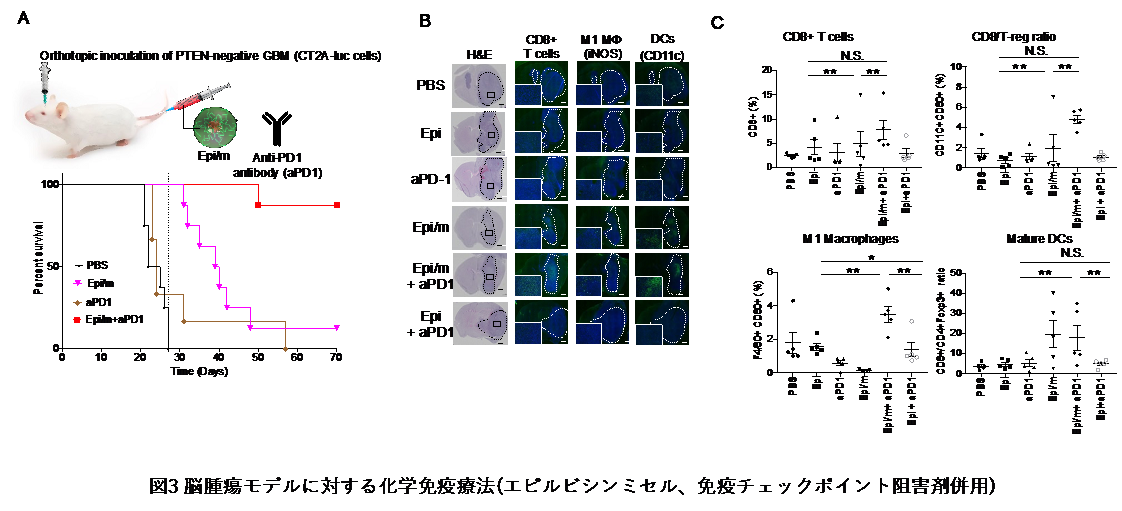
(ICI-resistant CT2A (PTEN-deficient strain)) orthotopic model was generated to test the superiority of chemoimmunotherapy with epirubicin micelles and ICI.(9) Similar to reports in brain tumor patients, the CT2A strain model lacking PTEN showed treatment resistance to ICI administration (aPD1 group). Epirubicin micelles accumulated 160-fold in tumor tissue compared to epirubicin alone, and when AUC was compared between normal and tumor brain tissue, it was 67-fold higher in tumor tissue, indicating that nanomicelle epirubicin brain migration was significantly higher in GBM tissue. Evaluation of survival by systemic administration showed that 90% of the combination therapy patients survived for more than 70 days, confirming a remarkable life-prolonging effect (Figure 3A). In addition, when the percentage of tumor-infiltrating T cells (TILs) was checked, it was found that combination therapy significantly increased the number of infiltrating T cells and M1 macrophage (activated macrophage) and dendritic cells (DCs) into the cancer, and conversely, decreased the number of bone marrow-derived immunosuppressed cells (MDSCs). The results showed that This chemoimmunotherapy with epirubicin micelles not only increased the proportion of patients who responded to ICI, but also proved to have an excellent synergistic effect in brain tumors not amenable to ICI, as demonstrated by the model.
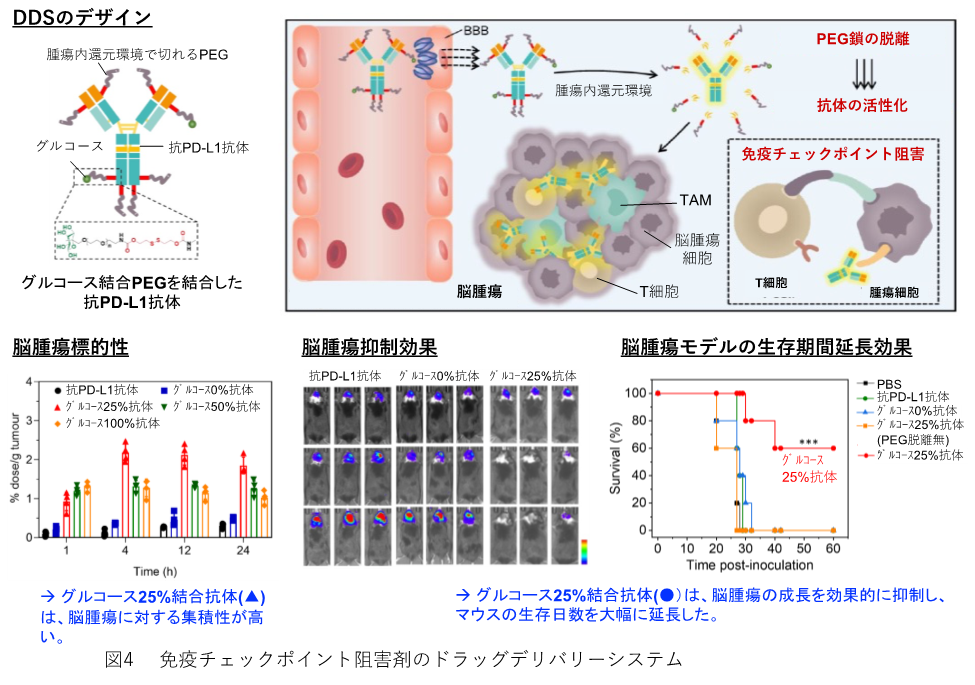
■Immune checkpoint inhibitor (ICI) delivery system to the brain
ICIs have a unique side effect called immune-related adverse events (irAE), as mentioned above. We have previously focused on glucose transporters expressed in vascular endothelial cells in the brain and have successfully induced glucose transporters by increasing blood glucose levels to deliver drugs to the brain via glucose-loaded micelles.(10) Recently, this glucose polymer and the reducing state in the cancer environment were successfully used to deliver immune checkpoint inhibitors to the brain (Figure 4). As shown in the figure, the ligand PEG polymer is bound to the antibody by S-S bonding to avoid nonspecific binding of proteins and other substances, circulates in the body, accumulates in the cancer by EPR effect, and the PEG polymer is desorbed in the reducing environment in the tumor and the antibody functions. The polymer with 25% glucose binding accumulated most in brain tumors and had the highest anti-tumor effect. We believe that the aforementioned ICD induction and the use of the immune checkpoint inhibitor DDS will lead to cancer treatment with fewer side effects.
brief personal record
-
Heisei 6
B.S. (Life Sciences), Graduate School of Natural Science and Technology, Kanazawa University
Doctor of Science -
1994
Research Associate, Cancer Research Institute, Kanazawa University
-
1996
Assistant Professor, Department of Biochemistry II, Faculty of Medicine, Toyama Medical and Pharmaceutical University
-
1997
Department of Cancer Cell Research, Institute of Medical Science, The University of Tokyo
Core Research Institutional Researcher -
Year 2000
Researcher, Daynabec Research Institute, Inc.
-
2006
Assistant Professor, Graduate School of Medicine, Chiba University Fellow
-
2010
Senior Researcher, Graduate School of Engineering, The University of Tokyo
-
Year ended March 31, 2015
Senior Researcher, Nano Medical Innovation Center, Planning and Technical Support Team Leader
performance
paper
- P. Wen, A. Dirisala, H. Guo, X. Liu, S. Kobayashi, H. Kinoh, T. Anada, M. Tanaka, K. Kataoka, J. Li Engineering durable antioxidative nanoreactors as synthetic organelles for autoregulatory cellular protection against oxidative stress. J Control Release (2025) DOI: 10.1016/j.jconrel.2025.113683
- Y. Honda, S. Nagao, H. Kinoh, X .Liu , N. Matsudaira, A. Dirisala, S. Nitta-Matsutomo , T. Nomoto, H. Hayashita-Kinoh, Y. Miura, T. Okada 5, N. Nishiyama Adeno-Associated Virus Self-Assembled with Tannic Acid and Phenylboronic Acid Polymers to Evade Neutralizing Antibodies and Reduce Adverse Events. ACS Nano. 2025 Mar 4;19(8):7690-7706. doi: 10.1021/acsnano.4c11085.
- Chen P, Yang W, Mochida Y, Li S, Hong T, Kinoh H, Kataoka K, Cabral H. Selective Intracellular Delivery of Antibodies in Cancer Cells with Nanocarriers Sensing Endo/Lysosomal Enzymatic Activity Angew Chem Int Ed Engl. (2024) Feb 11:e202317817. doi: 10.1002/anie.202317817.
- Mpekris F, Panagi M, Michael C, Voutouri C, Tsuchiya M, Wagatsuma C, Kinoh H, Osada A, Akinaga S, Yoshida S, Martin JD, Stylianopoulos T. J Control Release. (2023) Jan;353:956-964. doi: 10.1016/j.jconrel.2022.12.016.
- Y. Tao , Y. Mochida Y, X Liu, H. Zhou, J. Xie, H. Kinoh, H. Cabral, K. Kataoka. Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma. Nat. Biomed Eng. 5(11):1274-1287.(2021)
- J. Li, Z. Ge, K. Toh, X. Liu, A. Dirisala, W. Ke, P. Wen, Hang Zhou, Zheng Wang, Shiyan Xiao, Joachim F. R. Van Guyse, Theofilus A. Tockary, Jinbing Xie, Daniel Gonzalez‐Carter, H. Kinoh, Satoshi Uchida, Yasutaka Anraku, Kazunori Kataoka. Enzymatically Transformable Polymersome‐Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv Mater. 7:e2105254. (2021)
- W. Paraiso, J. G.-Chica, X. Ariza, S. Zagmutt, S. Fukushima, J. G. Gomez, Y. Mochida, D. Serra, L. Herrero, H. Kinoh, N. Casals, K. Kataoka, R. R.-Rodriguez, S. Quader, Poly-ion complex micelle effectively delivers CoA-conjugated CPT1A inhibitors to modulate lipid metabolism in brain cells. Biomater. Sci. 9(21):7076-7091. (2021)
- H. Shibasaki, H. Kinoh, H. Cabral, S. Quader, Y. Mochida, X. Liu, K. Toh, K. Miyano, Y. Matsumoto, T. Yamasoba, K. Kataoka. Efficacy of pH-Sensitive Nanomedicines in Tumors with Different c-MYC Expression Depends on the Intratumoral Activation Profile. ACS Nano. 15(3):5545-5559. (2021)
- S. Quader, X. Liu, K. Toh, Y.-L. Su, A. R. Maity, A. Tao, W. K. D. Paraiso, Y. Mochida, H. Kinoh, H. Cabral, K. Kataoka, Supramolecularly enabled pH- triggered drug action at tumor microenvironment potentiates nanomedicine efficacy against glioblastoma. Biomaterials 267 120463 (2021)
- H Cabral, H Kinoh, K Kataoka. Tumor-Targeted Nanomedicine for Immunotherapy. Acc Chem Res 53(12):2765-2776. (2020)
- H. Kinoh, S. Quader, H. Shibasaki, X. Liu, A. Maity, T. Yamasoba, H. Cabral, K. Kataoka, Translational nanomedicine boosts anti-PD1 therapy to eradicate orthotopic PTEN-negative glioblastoma. ACS Nano 14 Issue 8, 10127-10140 (2020)
- A. Dirisala, S. Uchida, K. Toh, J. Li, S. Osawa, T. A. Tockary, X. Liu, S. Abbasi, K. Hayashi, Y. Mochida, S. Fukushima, H. Kinoh, K. Osada, K. Kataoka, Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 6 (26) eabb8133 (2020)
- M. M. Lübtow, S. Oerter, S. Quader, E. Jeanclos, A. Cubukova, M. Krafft, C. Schulte, L. Meier, M. Rist, O. Sampetrean, H. Kinoh, A. Gohla, K. Kataoka, A. A.-Menzel, R. Luxenhofer, In vitro blood-brain-barrier permeability and cytotoxicity of atorvastatin-loaded nanoformulation against glioblastoma in 2D and 3D models. Mol. Pharm. 17 Issue6 1835-1847 (2020)
- H. Kinoh, H. Shibasaki, X. Liu, T. Yamasoba, H. Cabral, K. Kataoka, Nanomedicines blocking adaptive signals in cancer cells overcome tumor TKI resistance. J. Control. Release 321 132-144 (2020)
- T. A. Tockary, W. Foo, A. Dirisala, Q. Chen, S. Uchida, S. Osawa, Y. Mochida, X. Liu, H. Kinoh, H. Cabral, K. Osada, K. Kataoka, Single-stranded DNA-packaged polyplex micelle as adeno-associated-virus-inspired compact vector to systemically target stroma-rich pancreatic cancer. ACS Nano 13 Issue11 12732-12742 (2019)
- S. Watanabe, K. Hayashi, K. Toh, H. J. Kim, X. Liu, H. Chaya, S. Fukushima, K. Katsushima, Y. Kondo, S. Uchida, S. Ogura, T. Nomoto, H. Takemoto, H. Cabral, H. Kinoh, H. Tanaka, M. R. Kano, Y. Matsumoto, H. Fukuhara, S. Uchida, M. Nangaku, K. Osada, N. Nishiyama, K. Miyata, K. Kataoka, In vivo rendezvous of small nucleic acid drugs with charge-matched block catiomers to target cancer. Nat. Commun. 10 1894 (2019)
- W. Ke, J. Li, F. Mohammed, Y. Wang, K. Tou, X. Liu, P. Wen, H. Kinoh, Y. Anraku, H. Chen, K. Kataoka, Z. Ge, Therapeutic polymersome nanoreactors with tumor-specific activable cascade reactions for cooperative cancer therapy. ACS Nano 13 Issue 2 2357-2369 (2019)
- Y. Yi, H. J. Kim, M. Zheng, P. Mi, M. Naito, B S Kim, H. S. Min, K. Hayashi, F.Perche, K. Toh, X. Liu, Y. Mochida, H. Kinoh, H. Cabral, K. Miyata, K. Kataoka. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. Journal of controlled release 295: 268-277 (2019)
- J. Zhang, H. Kinoh, L. Hespel, X. Liu, S. Quader, J. Martin, T. Chida, H. Cabral, K. Kataoka, Effective treatment of drug resistant recurrent breast tumors harboring cancer stem-like cells by staurosporine/epirubicin co-loaded polymeric micelles. J. Control. Release 264 127-135 (2017)
- K. Miyano, H. Cabral, Y. Miura, Y. Matsumoto, Y. Mochida, H. Kinoh, K. Iwata, O. Nagato, H. Saya, N. Nishiyama, K. Kataoka, T. Yamasoba, cRGD peptide installation on cisplatin-loaded nanomedicines enhances efficacy against locally advanced head and neck squamous cell carcinoma bearing cancer stem-like cells. J. Control. Release 261 275-286 (2017)
- H. Kinoh, Y. Miura, T. Chida, X. Liu, K. Mizuno, S. Fukushima, Y. Morodomi, N. Nishiyama, H. Cabral, K. Kataoka, Nanomedicines eradicating cancer stem-like cells in vivo by pH-triggered intracellular cooperative action of loaded drugs. ACS Nano 10 (6) 5643-5655 (2016)
- S. Uchida S, H. Kinoh, T.Ishii, Matsui A, Tockary TA, Takeda KM, Uchida H, Osada K, Itaka K, K.Kataoka. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials Mar;82:221-8. (2016)
- J.Makino, H. Cabral, Y. Miura, Y. Matsumoto, M. Wang, H. Kinoh, Y. Mochida, N. Nishiyama, K. Kataok. cRGD-installed polymeric micelles loading platinum anticancer drugs enable cooperative treatment against lymph node metastasis. J Control Release. S0168-3659(15)30183-8. (2015)
- H. Wu, H. Cabral H, K. Toh , P Mi, YC Chen, Y. Matsumoto, N. Yamada, X. Liu, H. Kinoh, Y. Miura, Kano MR, H. Nishihara, N. Nishiyama, K. Kataoka. Polymeric micelles loaded with platinum anticancer drugs target preangiogenic micrometastatic niches associated with inflammation. J Control Release. 189:1-10. (2014)
- S. Quader, Cabral H, Mochida Y, Ishii T, Liu X, Toh K, H. Kinoh, Y. Miura, N. Nishiyama, K. Kataoka. Selective intracellular delivery of proteasome inhibitors through pH-sensitive polymeric micelles directed to efficient antitumor therapy. J Control Release. 188:67-77 (2014)
- Q Chen, K Osada, T Ishii , Oba M, Uchida S, Tockary TA, Endo T, Ge Z, H Kinoh, Kano MR, Itaka K, Kataoka K. Homo-catiomer integration into PEGylated polyplex micelle from block-catiomer for systemic anti-angiogenic gene therapy for fibrotic pancreatic tumors. Biomaterials. 33(18):4722-30. (2012)
- Y Morodomi Y, T Yano, H Kinoh, Y Harada, S Saito, R Kyuragi, K Yoshida, M Onimaru, F Shoji, T Yoshida, K Ito , Y Shikada, R Maruyam, M Hasegawa, Y Maehara , Y Yonemitsu. BioKnife, a uPA activity-dependent oncolytic Sendai virus, eliminates pleural spread of malignant mesothelioma via simultaneous stimulation of uPA expression. Mol Ther. 20(4):769-77 (2012)
- T Kato T, Y Ueda, H Kinoh, Y Yoneyama, A Matsunaga, A Komaru, Y Harada, H Suzuki, A Komiya, S Shibata , M Hasegawa, H Hayashi, T Ichikawa, Y Yonemitsu. RIG-I helicase-independent pathway in sendai virus-activated dendritic cells is critical for preventing lung metastasis of AT6.3 prostate cancer. Neoplasia. 12(11):906-14. (2010)
- Y Hasegawa, H. Kinoh, Y Iwadate, M Onimaru, Y Ueda, Y Harada, S Saito, A Furuya , T Saegusa, Y Morodomi, M Hasegawa, S Saito, I Aoki, N Saeki, Y Yonemitsu. Urokinase-targeted fusion by oncolytic Sendai virus eradicates orthotopic glioblastomas by pronounced synergy with interferon-β gene. Mol Ther. 18(10):1778-86 (2010)
- A Komaru, Y Ueda, A Furuya, S Tanaka, K Yoshida, T Kato, H Kinoh, Y Harada, H Suzuki, M Inoue, M Hasegawa, T Ichikawa, Y Yonemitsu. Sustained and NK/CD4+ T cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring recombinant Sendai virus. J Immunol 183(7): 4211-9. (2009)
- Y Harada, Y Ueda, H Kinoh, A Komaru, T Fuji-Ogawa, A Furuya, A Iida, M Hasegawa, T Ichikawa, Y Yonemitsu. Cytokine-based log-scale expansion of functional murine dendritic cells. PLoS One. 4(8): e6674. (2009)
- H Kinoh, M Inoue, A Komaru, Y Ueda, M Hasegawa, Y Yonemitsu. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 2009; 16(3): 392-403.
- H Inoue, Iga M, Nabeta, T Yokoo, Y Suehiro, S Okano, M Inoue, H Kinoh, T Katagiri, K Takayama, Y Yonemitsu, M Hasegawa, Y Nakamura, Y Nakanishi, K Tani Non-transmissible Sendai virus encoding granulocyte macrophage colony-stimulating factor is a novel and potent vector system for producing autologous tumor vaccines. Cancer Sci. 99(11):2315-26. (2008)
- Y. Yonemitsu, Y Ueda, H Kinoh, M Hasegawa. Immunostimulatory virotherapy using recombinant Sendai virus as a new cancer therapeutic regimen. Frontiers in bioscience : a journal and virtual library. Front Biosci. 13:1892-8. (2008)
- H Kinoh, Inoue M. New cancer therapy using genetically-engineered oncolytic Sendai virus vector. Front Biosci. 13:2327-34. (2008)
- Y Iwadate, M Inoue, T Saegusa, Y Tokusumi, H Kinoh, M Hasegawa, M Tagawa, A Yamaura, H Shimada. Recombinant Sendai virus vector induces complete remission of established brain tumors through efficient interleukin-2 gene transfer in vaccinated rats. Clin Cancer Res. 11(10):3821-7. (2005)
- H Kinoh, M Inoue, K Washizawa, T Yamamoto, S Fujikawa, Y Tokusumi, A Iida, Y Nagai, M Hasegawa. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases Gene Ther 11(14):1137-45. (2004)
- K Komori, T Nonaka, A Okada, H Kinoh, Hayashita-Kinoh H, Yoshida N, Yana I, Seiki M. Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett. 557(1-3):125-8. (2004)
- H Hayashita-Kinoh H, H Kinoh, A Okada, K Komori, Y Itoh, T Chiba, M Kajita, I Yana, M Seiki. Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differ 2001 Nov;12(11):573-80.
- M Kajita, Y Itoh, T Chiba, H Mori, A Okada, H Kinoh, M Seiki. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 153(5):893-904. (2001)
- Y Itoh, M Kajita, H Kinoh, H Mori, A Okada, M Seiki. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase J Biol Chem 274(48):34260-6. (1999)
- M Kajita 1, H Kinoh, N Ito, A Takamura, Y Itoh, A Okada, H Sato, M SeikiHuman membrane type-4 matrix metalloproteinase (MT4-MMP) is encoded by a novel major transcript: isolation of complementary DNA clones for human and mouse mt4-mmp transcripts FEBS Lett Sep 3;457(3):353-6. (1999)
- H Kinoh, H Hayashita, M Kajita, A Okada, M SeikiAssignment of the genes for membrane-type-4 matrix metalloproteinase (Mmp17, MMP17) to mouse chromosome 5, human chromosome band 12q24.3 and membrane-type-5 matrix metalloproteinase (Mmp24, MMP24) to mouse chromosome 2 and human chromosome band 20q11.2-->q12, respectively, by radiation hybrid and in situ hybridization. Cytogenet Cell Genet 97-8. (1999)
- K Wada, H Sato, H Kinoh, M Kajita, H Yamamoto, M Seiki. Cloning of three Caenorhabditis elegans genes potentially encoding novel matrix metalloproteinases. Gene 211(1):57-62. (1998)
- K Nomura, T Shimizu, H Kinoh, Y Sendai, M Inomata, N Suzuki. Sea urchin hatching enzyme (envelysin): cDNA cloning and deprivation of protein substrate specificity by autolytic degradation. Biochemistry 36(23):7225-38. (1997)
- Y Tsunezuka, H Kinoh, T Takino, Y Watanabe, Y Okada, A Shinagawa, H Sato, M Seiki. Expression of membrane-type matrix metalloproteinase 1 (MT1-MMP) in tumor cells enhances pulmonary metastasis in an experimental metastasis assay. Cancer Res 56(24):5678-83. (1996)
- H Kinoh, H Sato, Y Tsunezuka, T Takino, A Kawashima, Y Okada, M Seiki. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci 109 ( Pt 5):953-9. (1996)
- H Abe, H Kinoh, N Suzuki Seasonal variations in the production of the egg-jelly macromolecule, a fucose sulphate glycoconjugate, by the accessory cells in the ovary of the sea urchin Hemicentrotus pulcherrimus Rouxs Arch Dev Biol 402-410. (1994)
- H Kinoh, T Shimizu, H Fujimoto, N Suzuki. Expression of a putative precursor mRNA for sperm-activating peptide I in accessory cells of the ovary in the sea urchin Hemicentrotus pulcherrimus. Rouxs Arch Dev Biol 1994 Aug;203(7-8):381-388.
- Y Satoh 1, T Shimizu, Y Sendai, H Kinoh, N Suzuki. Nucleotide sequence of the proton ATPase beta-subunit homologue of the sea urchin Hemicentrotus pulcherrimus. Zoolog Sci 1994 Feb;11(1):153-6.
- H Abe, H Kinoh, T Oikawa, N Suzuki. The egg-jelly macromolecule, a fucose sulphate glycoconjugate, originates from the accessory cells of the ovary in the sea urchin Hemicentrotus pulcherrimus. Rouxs Arch Dev Biol 1992 May;201(3):179-189
- See https://iconm.kawasaki-net.ne.jp/kklab/research/research2-1je.html
patent
- JP2024-162530 (2024.9) Applicant:Kawasaki City Industrial Promotion Foundation Inventors:Hiroaki Kina, Thi Kim Dung Doan, Xueqing Liu, Sabina Kadar and Kazunori Kataoka [Title of the Invention]:Micelles for aqueous molecules and a method of delivering hydrophobic molecules using said micelles, and Compositions containing micelles encapsulating hydrophobic molecules for use in said methods
- JP2024-124555 (2024.7) Applicant: Kawasaki City Industrial Promotion Foundation, Kanazawa University, Kansai Medical University, etc. Inventors: Hiroaki Kina, Takeji Sakamoto, etc. Title of invention: Vaccine for treatment and prevention against hepatitis B virus
- JP2024-082791 (2024.10) Applicant: The University of Tokyo, Kawasaki City Industrial Promotion Foundation Inventors: Hiroaki Kina, Kanjiro Miyata, et al.
- Application No.: PCT/JP2023/017950, Title of invention: Medicine, kit and system Inventors: Hiroaki Kina, Kazunori Kataoka and 4 others, Applicant: Kawasaki City Industrial Promotion Foundation, Application date: May 13, 2022
- Application No.: PCT/JP2021/024675 Title of invention: Antibody with reduced binding affinity to antigen (to deliver full antibody (immunotick point inhibitor agent) Inventors: Kazunori Kataoka, Yasutaka Anraku, Yuki Mochida, Tao Yang, Cabral Horacio, Hiroaki Kinao
- Application No.: PCT/JP2020/021301, Title of the Invention: Complex, medicine, cancer treatment, kit and conjugate Inventors: Hiroaki Kinao, Kazunori Kataoka and 5 others, Applicant: Kawasaki City Industrial Promotion Foundation
- Invention title: Combination drug of micelle containing anthracycline compound and immunostimulant Inventors: Hiroaki Kinao, Kazunori Kataoka and 8 others; Applicant: Kawasaki City Industrial Promotion Foundation
- Application number: PCT/JP2019/006780, Title of invention: Pharmaceutical composition, Inventors: Hiroaki Kina, Kazunori Kataoka and six others, Applicant: Kawasaki City Industrial Promotion Foundation,
- Application No.: PCT/JP2019/006861, Title of invention: Pharmaceutical composition, Inventors: Hiroaki Kinao, Kazunori Kataoka and six others, Applicant: Kawasaki City Industrial Promotion Foundation
- Application No.: PCT/JP2017/30150, Title of the Invention: Polymer, Method of Making Polymer, and Drug Conjugate , Inventors: Hiroaki Kinao, Kazunori Kataoka and 2 others, Applicant: Kawasaki City Industrial Promotion Foundation, Application Date: 2019.2.22 Polymer, method for producing polymer, and drug conjugate Patent Application 2016-163134
- Title of invention: Pharmaceutical compositions for treating resistant and metastatic cancer in patients with resistant and metastatic cancer and containing staurosporine or its analogues
Applicant:The University of Tokyo Tokyo Institute of Technology
Inventors:Hiroaki Kinao Kazunori Kataoka Horacio Cabral Yutaka Miura Shigeto Fukushima - Title of invention: Cell fusion vector with modified protease-dependent tropism
Applicant: DNAVEC Laboratories, Inc.
Inventors: Hiroaki Kina, Makoto Inoue, Akihiro Iida, Mamoru Hasegawa - Title of the Invention: Gene transfer application of paramyxovirus vectors to blood vessels
Applicant: DNAVEC Laboratories, Inc.
Inventors: Ichiro Masaki, Yoshikazu Yonemitsu, Katsuo Iishi, Mamoru Hasegawa, Hiroaki Kinao - Title of the Invention: Viral Vectors for Gene Therapy
Applicant: Kyushu University, DNAVEC Corporation
Inventors: Kensaburo Tani, Mamoru Hasegawa, Makoto Inoue, Hiroaki Kinao
Academic Affiliations
- Councilor, Japanese Society for Gene and Cell Therapy
- The Japanese Cancer Association
- Japan DDS Society
- The Japanese Society for Biomaterials
- The Molecular Biology Society of Japan
- Japan Respiratory Society
- The Japanese Association for Cancer Immunology
- The Japanese Association for Molecular Targeting of Cancer
Research funds approved and received to date
Japan Agency for Medical Research and Development Basic drug discovery technology development project for realization of next-generation treatment and diagnosis: Research and development of pharmaceuticals using next-generation delivery technology Representative (R6-R11) Subject name: Development of the world's first cancer curing technology by effective tumor targeting using innovative ligand-directed multi-target siRNA and nanomachine complex
Grant-in-Aid for Scientific Research, Basic Research (B), Representative (R6~ R9, MEXT) Title: Development of targeted therapy for pulmonary fibrosis (IPF) using DDS system with ligand nucleic acid medicine
Japan Agency for Medical Research and Development, Next-Generation Accelerated Cancer Therapy Research Project, Research Project (R5-R6) Title: Development of Innovative Immune Checkpoint Inhibitors Based on Polymer Modification of Antibodies
Grant-in-Aid for Scientific Research (B) (R4~ R6) Title: Creation of unstable iron chelation technology to transform cold tumor into hot tumor
Grant-in-Aid for Scientific Research, Basic Research (C), Research Subject (R4~ R6) Title:Nanomedicine-based Chemo-Immunotherapy for the Treatment of Pediatric Brain Cancer
Grant-in-Aid for Scientific Research, Scientific Research (C), Dependent on (R4~ R6) Subject name:Development of immune response evading drugs for gene therapy of Duchenne muscular dystrophy
Fund for Accelerating Global Research Collaboration (Strengthening Global Research Collaboration (B)) (R3~ R6) Subject name:Development of nanotherapeutics to boost immunotherapy by precisely targeting cancer-related fibroblast cells
Japan Agency for Medical Research and Development, Fundamental Technology Development Project for Advanced Biotechnology and Drug Discovery (H31~ R5) Subject name:Development of biopharmaceutical delivery systems based on polymer nanotechnology
Grant-in-Aid for Scientific Research, Basic Research (B) Representative Title:Development of nanotherapeutics to overcome resistance to immune checkpoint inhibitors by tumor stroma in refractory cancer
Grant-in-Aid for Scientific Research, Basic Research (C) Representative Title:Development of novel nanomicelles targeting therapy-resistant cancer stem cells in refractory cancer that recurs